
Let’s compare atoms in Group 2: the alkaline earth metals.
Just to review, groups on the periodic table are just columns. Several methods of calculation have been proposed, and although there may be small differences in the numerical values of the electronegativity, all methods show the same periodic trends between elements. Electronegativity cannot be directly measured and must be calculated from other atomic or molecular properties. It has been shown to correlate with a number of other chemical properties. In spite of its long history, an accurate scale of electronegativity was not developed until 1932, when Linus Pauling proposed an electronegativity scale, which depends on bond energies, as a development of valence bond theory. The term “electronegativity” was introduced by Jöns Jacob Berzelius in 1811, though the concept was known even before that and was studied by many chemists including Avogadro.
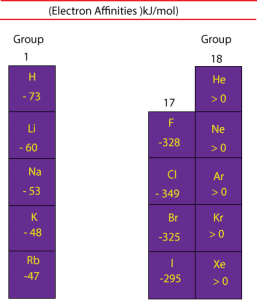
On the most basic level, electronegativity is determined by factors like the nuclear charge (the more protons an atom has, the more “pull” it will have on electrons) and the number/location of other electrons present in the atomic shells (the more electrons an atom has, the farther from the nucleus the valence electrons will be, and as a result the less positive charge they will experience-both because of their increased distance from the nucleus, and because the other electrons in the lower energy core orbitals will act to shield the valence electrons from the positively charged nucleus). The higher the associated electronegativity number, the more an atom or a substituent group attracts electrons towards itself. An atom’s electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The chemical rationale for changes in electron affinity across the periodic table is the increased effective nuclear charge across a period and up a group.Įlectron affinity: The electron affinity of an atom or molecule is defined as the amount of energy released when an electron is added to a neutral atom or molecule to form a negative ion.Įlectronegativity: The tendency of an atom or molecule to attract electrons to itself.Electronegativity Trend | What Is Electronegativity Trend?Įlectronegativity Trend, symbol χ, is a chemical property that describes the tendency of an atom to attract a shared pair of electrons (electron density) towards itself.Electron affinity generally increases across a period in the periodic table and sometimes decreases down a group. There are general trends in electron affinity across and down the periodic table of elements.The electron affinity of an atom or molecule is the propensity for that particle to gain an electron.Electron affinity follows the trend of electronegativity: fluorine (F) has a higher electron affinity than oxygen (O), and so on.Īpplications of Hard-Soft Acid-Base theoryĬhemistry Question Pack Passage 1 Question 4 However, this trend applies only to Group-1 atoms. Since this electron is farther away, it should be less attracted to the nucleus and release less energy when added. A trend of decreasing electron affinity down the groups in the periodic table would be expected since the additional electron is entering an orbital farther away from the nucleus. Chlorine has the highest electron affinity while mercury has the lowest.Įlectron affinity generally increases across a period (row) in the periodic table, due to the filling of the valence shell of the atom. The electron affinities of the noble gases have not been conclusively measured, so they may or may not have slightly negative values. Atoms, such as Group 7 elements, whose anions are more stable than neutral atoms have a higher electron affinity. Generally, nonmetals have a more positive electron affinity than metals. This can be shown for the chloride ion formation below:

The electron affinity (E ea) of a neutral atom or molecule is defined as the amount of energy released when an electron is added to it to form a negative ion. Electron affinity is the energy change that occurs when a neutral atom gains an electron.


 0 kommentar(er)
0 kommentar(er)
